Syngas production: diverse H2/CO range by regulating carbonates electrolyte composition from CO2/H2O via co-electrolysis in eutectic molten salts†
Abstract
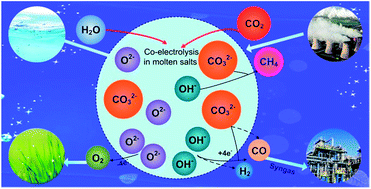
We present a novel sustainable method for the direct production of syngas (H2 + CO) from CO2/H2O co-electrolysis using a hermetic device, to address the continuously increasing level of environmental carbon dioxide (CO2). All experiments were conducted using a two-electrode system with a coiled Fe cathode and coiled Ni anode in eutectic mixtures of binary and ternary carbonates with hydroxide in a 0.1 : 1 hydroxide/carbonate ratio. With an applied voltage of 1.6–2.6 V and an operating temperature of 500–600 °C, the H2/CO product ratio was easily tuned from 0.53 to 8.08 through renewable cycling of CO2 and H2O. The Li0.85Na0.61K0.54CO3–0.1LiOH composite had the highest current efficiency among those tested, with an optimum value approaching ∼93%. This study provides a promising technique for the electrochemical conversion of CO2/H2O to a controllable syngas feedstock that can be used in a broad range of industrial applications.


 Please wait while we load your content...
Please wait while we load your content...