Preparation of cationic polymeric nanoparticles as an effective adsorbent for removing diclofenac sodium from water
Abstract
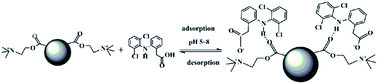
New cationic polymeric nanoparticles (PNPs) were synthesized by precipitation polymerization using [2-(methacryloyloxy)ethyl] trimethylammonium chloride and ethylene glycol dimethacrylate for adsorption of diclofenac sodium. The PNPs were characterized using FTIR, SEM, TEM, and their specific surface areas and thermal stabilities were measured. The adsorption capacity of PNPs was studied by static adsorption experiments. The adsorption equilibrium was achieved within only 7 min, and quantitative desorption required 3 min using 0.1 mol L−1 NaOH/methanol (1 : 1, v/v) as eluent. The maximum adsorption capacity of the PNPs for diclofenac sodium was 334.2 mg g−1. The specific surface area for the PNPs was 192.5 m2 g−1. The isothermal adsorption model and the kinetic model were well fitted with the Langmuir adsorption model and a pseudo-second order kinetic model, and the adsorption mechanism was also discussed in detail. The PNPs could be used repeatedly for 6 cycles without a significant decrease in adsorption capacity. The prepared PNPs can be considered to be a promising sorbent for the adsorption of diclofenac sodium from water samples.


 Please wait while we load your content...
Please wait while we load your content...