Confinement of alcohols to enhance CO2 capture in MIL-53(Al)†
Abstract
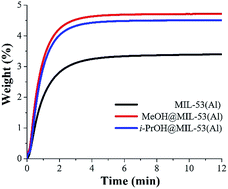
CO2 capture of MIL-53(Al) was enhanced by confining MeOH and i-PrOH within its micropores. Compared to MIL-53(Al), results showed an approximately 1.3 fold increase in CO2 capture capacity (kinetic isothermal CO2 adsorption experiments), via confining small amounts of both alcohols. Adsorption–desorption properties are investigated for MeOH and i-PrOH and the enthalpy of adsorption, for MeOH and i-PrOH, was measured by differential scanning calorimetry (DSC): ΔH = 50 and 56 kJ mol−1, respectively. Regeneration (CO2 adsorption–desorption cycles) of the sample MeOH@MIL-53(Al) exhibited a loss on the CO2 capacity of only 6.3% after 10 cycles and the desorption is accomplished by only turning the CO2 flow off. Static CO2 adsorption experiments (at 196 K) demonstrated a 1.25-fold CO2 capture increase (from 7.2 mmol g−1, fully activated MIL-53(Al) to 9.0 mmol g−1, MeOH@MIL-53(Al)). The CO2 enthalpy of adsorption for MIL-53(Al) and Me@OHMIL-53(Al) were estimated to be ΔH = 42.1 and 50.3 kJ mol−1, respectively. Computational calculations demonstrated the role of the hydrogen bonds formed between CO2 molecules and confined MeOH and i-PrOH molecules, resulting in the enhancement of the overall CO2 capture.


 Please wait while we load your content...
Please wait while we load your content...