Competitive biosorption behavior of Pt(iv) and Pd(ii) by Providencia vermicola
Abstract
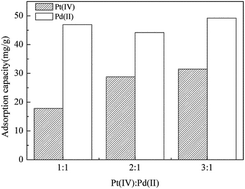
Biosorption is an effective way to recover or remove metal ions from wastewater; however, the biosorption process in a multiple metal ion solution is still unclear. In this paper, we investigated the simultaneous biosorption in a Pt(IV) and Pd(II) binary system using Providencia vermicola. Kinetics, isotherms, thermodynamics, TEM, and FT-IR were carried out to elucidate the competition behavior and adsorption mechanisms in the process. The results revealed that Pd(II) had a relevant effect on the Pt(IV) biosorption, but the interference of Pt(IV) on the adsorption of Pd(II) was considerably less intense. The maximum adsorption capacity of P. vermicola was 30.20 mg g−1 for Pt(IV) and 119.00 mg g−1 for Pd(II) in the binary system, which corresponded to 3.94 times larger selective adsorption toward Pd(II). The TEM results revealed that P. vermicola could adsorb Pt(IV) and Pd(II) both extracellularly and intracellularly. The FT-IR results indicated that amine groups played major roles in adsorbing Pt(IV), but amine, carboxyl, hydroxyl, and phosphate groups were critical to adsorb Pd(II). This study demonstrates that P. vermicola is a promising biosorbent and could selectively adsorb Pd(II) in the Pt(IV)–Pd(II) binary system.


 Please wait while we load your content...
Please wait while we load your content...