A turn-on green channel Zn2+ sensor and the resulting zinc(ii) complex as a red channel HPO42− ion sensor: a new approach†
Abstract
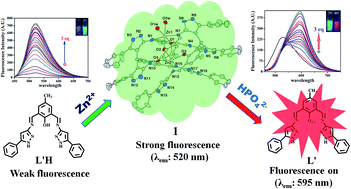
A newly synthesized and spectroscopically characterized non-fluorescent organic moiety (L′H) selectively sensed Zn2+ ions based on a chelation-enhanced fluorescence (CHEF) process at the λem of 520 nm through the formation of a new dinuclear zinc(II) complex (1). Upon the addition of Zn2+ ions to the solution of L′H in dimethyl sulphoxide at 25 °C, a systematic enhancement of fluorescence was observed, which was not affected by the presence of competitive ions. The reaction of L′H with Zn2+ ions led to the formation of a dinuclear zinc(II) complex, featuring a new in situ formed macrocyclic ligand (L), which was isolated in pure form and then characterized. The formulation of 1 was established by spectroscopic tools along with a detailed structural analysis carried out using single crystal X-ray crystallography. In addition, the complex 1 also behaved as a red-shifted, HPO42− ion-selective chemosensor at the λem of 595 nm based on a displacement approach in dimethyl sulphoxide. Interestingly, 1 showed remarkable sensitivity towards HPO42− ions via fluorescence modulation of the dinuclear zinc(II) complex (1) compared with the other anions examined herein.


 Please wait while we load your content...
Please wait while we load your content...