Highly selective hydrodeoxygenation of anisole, phenol and guaiacol to benzene over nickel phosphide†
Abstract
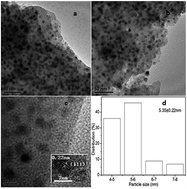
Ni2P supported catalysts have extensively been studied for various hydrodeoxygenation (HDO) reactions. However, the main products are cyclohexane or cyclohexanol for lignin-derived compounds HDO over these catalysts. In this study, we investigate the catalytic conversion of anisole, phenol and guaiacol to benzene over Ni2P/SiO2 by probing the reaction conditions. The results show that a lower reaction temperature and higher H2 pressure favour the hydrogenation of these model chemicals to cyclohexane, whereas a higher reaction temperature and lower H2 pressure aid the generation of benzene. The cyclohexane and benzene yields are 89.8% and 96.0% at 1.5 MPa and 573 K and 0.5 MPa and 673 K, respectively. By eliminating the influence of internal and external diffusion, the low intrinsic activation energy of 58.2 kJ mol−1 is obtained, which explains the high catalytic activity. In addition, although guaiacol HDO has a low conversion due to the space steric effect of its substituents, it presents a similar reaction pathway to obtain anisole and phenol, which is dependent on reaction conditions. The long-run evaluation experiment shows that the activity and selectivity of anisole HDO to benzene changes slightly for 36 h.


 Please wait while we load your content...
Please wait while we load your content...