Synthesis, structures and catalytic activity of cyclometalated rhenium complexes†
Abstract
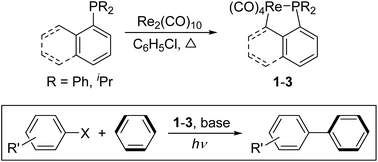
Thermal reactions of aryl-substituted phosphines or phosphinites with Re2(CO)10 in chlorobenzene resulted in the corresponding five-membered cyclometalated rhenium complexes (1–5) via an intramolecular activation of the C(sp2)–H or C(sp3)–H bond. The only exception occurred in the case of (1-naphthyl)diisopropylphosphinite, which gave a diphosphinite-substituted dinuclear rhenium complex 6. Competition reaction indicated that the aromatic C(sp2)–H bond is more likely to be activated than the C(sp3)–H bond under the same conditions. Photolysis of 1 or 2 in CHX3 led to the cleavage of the Re–C σ bond to yield corresponding phosphine-substituted tetracarbonyl rhenium halides 7–10. Complex 1 reacted with CF3COOH in CH2Cl2 to give addition product 11. Photolysis of cyclorhenated complexes 1–3 with a series of aryl halides in benzene resulted in the stoichiometric formation of biphenyl, together with corresponding phosphine-substituted tetracarbonyl rhenium halides (7, 9, 10, 12, and 13). When base was introduced into the above reaction, a catalytic system was established. Under optimized conditions, complex 1 provided moderate yield of biphenyl in a couple of hours at a [Re] : substrate ratio of 1 : 200. Molecular structures of complexes 1, 6, 9, and 11 were determined by X-ray diffraction.


 Please wait while we load your content...
Please wait while we load your content...