Investigation of pseudo-polyanion formation between polyvinylpyrrolidone and sodium dodecanoate in aqueous solution by capillary electrophoresis, conductometry, tensiometry and calcium stability
Abstract
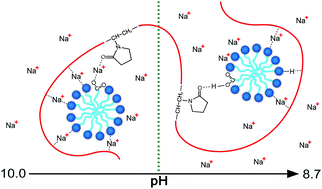
There is a lack of information about association between nonionic polymers and weak electrolyte type anionic surfactants, therefore the complexation between polyvinylpyrrolidone (PVP) and sodium dodecanoate (SD) is investigated by capillary electrophoresis for the first time, in assistance with conductometry, tensiometry and calcium stability. The experimental results show that values of critical aggregation concentration (cac) and polymer saturation point (psp) of the system determined by several methods are consistent with each other. It is firstly found that the PVP–SD complex is actually PVP–SD pseudo-polyanions with different charge densities corresponding to different pH levels. It is also found that H-bonding and counterion bridging between PVP and bound SD micelles play important roles in the self-assembling of the PVP–SD pseudo-polyanions, and the pH-dependent capillary electrophoresis behavior of the pseudo-polyanions is ascribed to sodium bridging being replaced by H-bonding at lower pH.


 Please wait while we load your content...
Please wait while we load your content...