Investigation of oxygen self-diffusion in PuO2 by combining molecular dynamics with thermodynamic calculations
Abstract
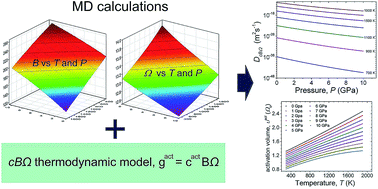
In the present work, the defect properties of oxygen self-diffusion in PuO2 are investigated over a wide temperature (300–1900 K) and pressure (0–10 GPa) range, by combining molecular dynamics simulations and thermodynamic calculations. Based on the well-established cBΩ thermodynamic model which connects the activation Gibbs free energy of diffusion with the bulk elastic and expansion properties, various point defect parameters such as activation enthalpy, activation entropy, and activation volume were calculated as a function of T and P. Molecular dynamics calculations provided the necessary bulk properties for the proper implementation of the thermodynamic model, in the lack of any relevant experimental data. The estimated compressibility and the thermal expansion coefficient of activation volume are found to be more than one order of magnitude greater than the corresponding values of the bulk plutonia. The diffusion mechanism is discussed in the context of the temperature and pressure dependence of the activation volume.


 Please wait while we load your content...
Please wait while we load your content...