Effect of pH and temperature on conformational equilibria and aggregation behaviour of curcumin in aqueous binary mixtures of ethanol†
Abstract
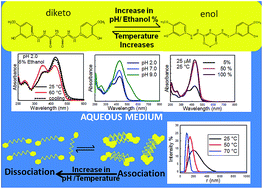
Recently, curcumin has emerged as a potential therapeutic agent against many chronic diseases, due to some of its significant biological properties. However, the mechanism of its action is not properly understood, due to the lack of physicochemical knowledge on this compound in aqueous media. In the present article, we have investigated the effect of pH and temperature on the conformational equilibria and aggregation behavior of curcumin in an almost completely aqueous solution and in solutions containing different binary mixtures of ethanol and water. Keto formation was favored at acidic pH, whereas neutral and anionic forms of curcumin were predominant at neutral and alkaline pH, respectively. Moreover, the curcumin solutions were found to be more heterogenous at acidic and neutral pH, due to the presence of different aggregated forms of curcumin, as characterized by fluorescence and DLS studies. Temperature was also found to significantly influence the keto–enol–enolate equilibrium, as well as the aggregation of curcumin. Keto–enol tautomerization was observed to be enthalpy driven at both acidic and neutral pH.

 Please wait while we load your content...
Please wait while we load your content...