Effect of Mn doping on the activity and stability of Cu–SiO2 catalysts for the hydrogenation of methyl acetate to ethanol†
Abstract
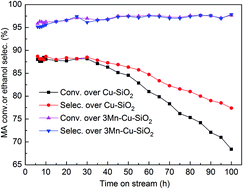
A series of xMn–Cu–SiO2 catalysts with different manganese contents were prepared by an ammonia-evaporation method for methyl acetate hydrogenation. The activity and stability of the catalysts were greatly improved when manganese content was 3%. Besides, physicochemical properties of these catalysts were investigated by N2 physisorption, X-ray diffraction, H2-temperature programmed reduction and X-ray photoelectron spectroscopy. The results illustrated that doping a suitable amount of manganese onto silica-supported copper catalysts produced a strong interaction between cupreous species and Mn, diminished the copper crystalline size, enlarged the copper surface area and enriched the surface Cu+ species, so as to improve the catalytic activity and stability of the 3Mn–Cu–SiO2 catalyst.

 Please wait while we load your content...
Please wait while we load your content...