Enhancement of mass transfer through bubbling effect during extraction and reaction in biphasic systems containing ionic liquid
Abstract
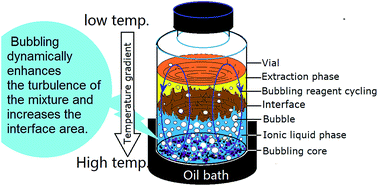
Separation of a compound from an ionic liquid through extraction usually results in a low mass transfer rate because of the high viscosity of the ionic liquid and the large density difference between the ionic liquid and organics even under strong stirring. An improved interface mass transfer during extraction or biphasic reaction was realized by creating a bubbling effect in a biphasic system containing an ionic liquid. In a model biphasic system containing 1-butyl-3-methylimidazolium chloride ([BMIM]Cl) as the solvent phase and methyl isobutyl ketone (MIBK) as the extraction phase, the bubbling reagents partitioned in the [BMIM]Cl phase, such as methanol, ethanol and acetonitrile, exhibit dynamic equilibrium between the two phases through constant gasification from and dissolution into the [BMIM]Cl phase. Bubbling increases the interface area and dynamically enhances the turbulence of the mixture; both phenomena are beneficial for fast transfer of 5-hydroxymethylfurfural from the ionic liquid phase to the extraction phase. The organic solvents suitable as bubbling reagents were identified, and the key parameters in establishing bubbling biphasic systems were determined.

 Please wait while we load your content...
Please wait while we load your content...