Nitrogen induced phosphorene formation on the boron phosphide (111) surface: a density functional theory study†
Abstract
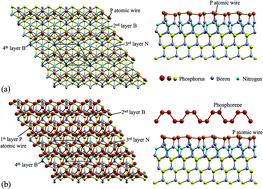
Nitrogen induced phosphorene formation on top of the BP (111) surface is investigated using periodic density functional theory (DFT) calculations. We have considered adsorption/incorporation of different amounts of N, from 1/4 to 1 monolayer. Results demonstrate that the incorporation of a full N monolayer into the second P monolayer drives the formation of a P atomic wire at the surface. Adsorption of two monolayers on top of the phosphorus atomic wire results in the formation of a phosphorene atomic structure. Surface formation energy calculations indicate that for most of the allowed chemical potential range, the structure with the P zigzag atomic wire array is stable. However, for phosphorus rich conditions, the formation of a phosphorene configuration is also possible with almost the same formation energy. The phosphorene structure is bonded by van der Waals forces to the P atomic wire resembling the bilayer–bilayer distance in black phosphorus. From these results, it is clear that the N incorporation plays a key role in the stability and formation of phosphorene.

 Please wait while we load your content...
Please wait while we load your content...