Alpha and beta diimine cobalt complexes in isoprene polymerization: a comparative study†
Abstract
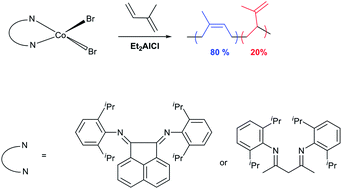
Two cobalt complexes, the α-diimine complex 1,2-bis[(2,6-diisopropylphenyl)imino]acenaphthene-cobalt(II) dibromide (A) and the β-diimine complex 2,4-bis[(2,6-diisopropylphenyl)imino]pentane-cobalt(II) dibromide (B) were synthesized and fully characterized by elemental analysis, electrospray MS and single crystal X-ray diffraction. These complexes were then assessed as pre-catalysts of the polymerization of isoprene in chlorobenzene using diethylaluminiumchloride (DEAC) as co-catalyst at 35 °C. While the aryl substituents were identical in each case, the β-diimine complex B was over 100-fold more active (Rp = 1.17 × 10−3 M s−1) than α-diimine complex A (Rp = 1.07 × 10−5 M s−1). A produced polyisoprene with molecular weight (Mn = 2.1 × 105 g mol−1) double that achieved with B. The polymers produced by both A and B were predominantly cis-1,4 enchained (80%) with 3,4 regioerrors (20%). For B, this 1,4 : 3,4 selectivity ratio was not influenced by aluminium alkyl reagent (ethylaluminiumsesquichloride (EASC), or DEAC) whereas for A, where EASC co-catalyst was used, a 1,4-trans-rich (61%) polyisoprene was obtained. Moreover, increasing Al mole ratio decreased the activity for B but increased it for A.

 Please wait while we load your content...
Please wait while we load your content...