β,β-Dialkyl γ-amino γ-trifluoromethyl alcohols from trifluoromethyl (E)-aldimines by a one-pot solvent-free Mannich-type reaction and subsequent reduction†
Abstract
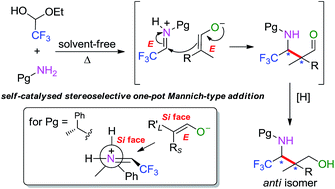
The synthesis of trifluoromethylated γ-amino alcohols through an eco-friendly one-pot self-catalysed Mannich-type reaction between N-protected trifluoromethyl aldimines and suitable cyclic or acyclic α,α-dialkyl aldehydes has been developed. Good yields, mild reaction conditions and simple experimental work-up procedures are some of the advantages of this method. Starting from optically pure trifluoromethyl aldimines, target compounds, also having a quaternary stereocentre, can be obtained in good to excellent diastereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...