Regioselective synthesis of spiroxindolopyrrolidine: a one step cycloaddition reaction twists inherent optical and fluorescence property of ferrocene–anthracene dyad†
Abstract
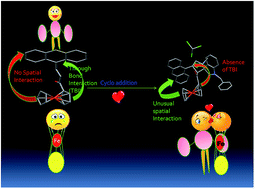
We report a regioselective synthesis of spiroxindole using a 1,3-dipolar cycloaddition reaction (1,3-DC). The ferrocene–anthracene (Fc–An) dyad is used as the dipolarophilic partner. After the introduction of oxindole by cycloaddition, the fluorescence of anthracene is surprisingly enhanced. Using 1H NMR, we have observed an unusual up field shift of the ferrocene aromatic protons from δ 4.2 to δ 2.87, which reveals an interaction between the ferrocene and anthracene π systems. These novel interactions are observed only after the regioselective cycloaddition on the Fc–An core. Our results shows that cycloaddition can alter the inherent optical and fluorescence properties of the Fc–An dyad system.

 Please wait while we load your content...
Please wait while we load your content...