Quantitative chemical relations at pseudo-equilibrium in amorphous calcium phosphate formation†
Abstract
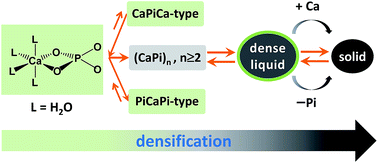
Amorphous calcium phosphate (ACP) is a precursor to crystalline hydroxyapatite, and has been found to be present in zebrafish and mouse bone formation. Most of the previous studies on ACP formation are qualititative in nature. Here, we report a quantitative correlation between the solution composition and the amount of ACP. Solutions with the initial Ca/P molar ratios between 0.5 and 1.5 were made either by fixing calcium and varying phosphate concentration (Series 1), or by fixing phosphate and varying calcium concentration (Series 2). From the two reaction series, we derived pseudo-equilibrium equations, and determined the threshold concentrations for ACP formation and its maximum amount, which are present as constants in these equations. Based on these results, we proposed a mechanism for ACP formation and derived the formation constant that depends on both the solution composition and the ACP amount. This work might represent an advance toward understanding the basic aspects of solution chemistry involving clusters and an amorphous phase of calcium phosphate, and the “two series” approach could be applicable to the systematical study of the amorphous phase of other sparingly soluble electrolytes. Moreover, these findings might provide new insight into the relevant physiological phenomena and be helpful for the rational design of functional materials.


 Please wait while we load your content...
Please wait while we load your content...