Rate acceleration for 4,4′-dimethoxydiphenyl nitroxide mediated polymerization of methyl methacrylate†
Abstract
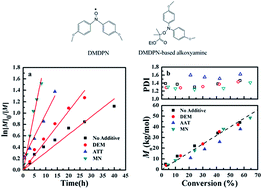
An organic acid, camphorsulfonic acid (CSA) and three strong polar compounds, diethyl malonate (DEM), acetylacetone (AAT) and malononitrile (MN) were used to accelerate the polymerization of methyl methacrylate (MMA) mediated by 4,4′-dimethoxydiphenyl nitroxide (DMDPN). Polymerization with CSA proceeded quite fast but was not “living”. When DEM, AAT and MN were used as accelerators, MN supplied the best results. A conversion of 45% was achieved in 3 h with an optimal [MN]/[alkoxyamine] molar ratio of 3 : 1 and the dispersity index was even lower than that without any additive. Electron spin resonance (ESR) study showed the NO–C bond could be weakened by MN, resulting in a slightly increase in kd. Both of the weakening of the NO–C bond and the dipole–dipole interaction between MN and DMDPN attribute to the rate acceleration, while the dipole–dipole interaction plays the pivotal role.


 Please wait while we load your content...
Please wait while we load your content...