Synthesis and evaluation of mesopore structured ZSM-5 and a CuZSM-5 catalyst for NH3-SCR reaction: studies of simulated exhaust and engine bench testing†
Abstract
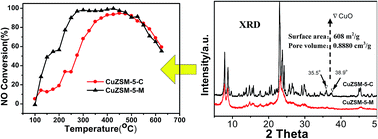
A modified ZSM-5 zeolite (denoted as ZSM-5-M), which was synthesized using tetrapropylammonium hydroxide (TPAOH) and cetyltrimethylammonium bromide (CTAB) as dual templates, and the commercial ZSM-5 zeolite (denoted as ZSM-5-C), have been used to prepare the corresponding CuZSM-5 (M & C) catalysts containing 3 wt% Cu by ion exchange method. Compared to CuZSM-5-C catalyst, the CuZSM-5-M catalyst demonstrated remarkably higher catalytic activity at low temperatures (<450 °C) for selective catalytic reduction of NOx with NH3 both in the simulated exhaust and engine bench testing. The modified synthesis by the dual template with the product aged for a long time at room temperature, leads to the formation of the ZSM-5-M zeolite with much higher specific surface area (608 m2 g−1) and higher total pore volume (0.8880 cm3 g−1) due to the presence of more mesoporous pores. The X-ray diffraction results showed that ZSM-5-M maintained its typical MFI structure, while its crystallinity (84.1%) was lower than that of the ZSM-5-C zeolite. The characterization results by H2 temperature-programmed reduction and X-ray photoelectron spectra revealed that the higher redox properties of isolated Cu2+ ions combined with the high-dispersion CuO crystallites and Cu+ ions are likely the main cause for the excellent low-temperature activity of the CuZSM-5-M catalysts. The isolated active Cu2+ species and high-dispersion CuO crystallites had a stronger interaction with other atoms in CuZSM-5-M catalysts. The results from thermogravimetric analysis, temperature-programmed desorption of ammonia and in situ diffuse reflectance infrared Fourier transform spectroscopy demonstrated that ZSM-5-M is a strong Brønsted acid site and the CuZSM-5-M catalyst had a relatively higher exchange rate of Cu2+ and Cu+ ions and more Lewis acidic sites, giving it a high NH3 adsorption capacity. The strong Brønsted acid site might be another cause that results in the higher NH3-SCR performance of the CuZSM-5-M catalyst.

 Please wait while we load your content...
Please wait while we load your content...