Structure-dependent electronic transition in a new type of π-electron delocalized multi-sulfur bis(dithiolene)nickel complex†
Abstract
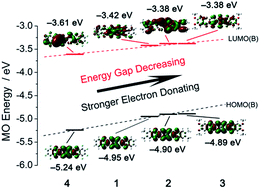
Four new nickel bis(dithiolene) complexes with different substituent groups on their benzene rings were synthesized and characterized. X-ray structure analysis reveals that the anions of these Ni-complexes exhibit chair-like configurations. Magnetic measurements confirm that compounds 1–4 show antiferromagnetic interactions. The absorption spectra show strong and broad absorptions in the near-infrared region (700–1100 nm). Density functional theory (DFT) calculations indicate that the HOMO and LUMO energy levels are highly dependent on the electron donating/withdrawing abilities of the substituents. The electron-donating substituent results in a bathochromic-shift absorption, while the electron-withdrawing substituent leads to a hypsochromic-shift absorption band. Moreover, the results of solution-state electrochemical studies also show a good agreement with our findings in spectroscopic and DFT studies.


 Please wait while we load your content...
Please wait while we load your content...