Facile synthesis of an Fe3O4/FeO/Fe/C composite as a high-performance anode for lithium-ion batteries
Abstract
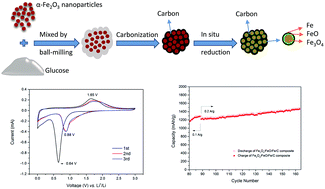
Fe3O4/FeO/Fe nanoparticles coated with amorphous carbon are prepared via a facile and scalable in situ-reduction solid synthesis route as high-performance anode material for lithium ion batteries (LIBs). The amorphous carbon coating is produced by carbonization of glucose in the precursor, leading to partial reduction of Fe3+ in situ and a tight intermolecular contact between Fe3O4/FeO/Fe nanoparticles and the carbon layer. The formation of Fe in the composite dramatically increases the conductivity of the electrode, making a significant contribution to the enhancement of electrochemical performance. When used as anode materials in lithium ion batteries, the as-prepared Fe3O4/FeO/Fe/C composite shows super high rate capability (685, 545, and 407 mA h g−1 at 2, 5, and 10C, respectively, 1C = 1 A g−1) and excellent cycling performance (900 mA h g−1 after 500 cycles at 1C).

 Please wait while we load your content...
Please wait while we load your content...