The self-assembly mechanism of tetra-peptides from the motif of β-amyloid peptides: a combined coarse-grained and all-atom molecular dynamics simulation†
Abstract
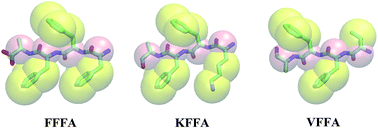
Understanding the self-assembly mechanisms of peptides into nanostructures is essential for the rational design of bio-nanomaterials. Moreover, the natural fiber formation of Alzheimer’s β-amyloid peptides is crucially involved in Alzheimer’s disease but the mechanism still remains obscure. Herein, the assembly of the tetra-peptide motif VFFA from Aβ peptides and its derivations KFFA and FFFA into different nanostructures was investigated with combined coarse-grained (CG) and all-atom (AA) models. The primary structures of the tetra-peptides were found to be the most important factor to form special nanostructures rather than the concentration of the tetra-peptides. FFFA tends to form nanosheets, while VFFA tends to form nanospheres and KFFA tends to form nanorods from the CG simulation. The stabilities of the aggregated structures from the CG simulation were investigated and confirmed by AA simulations. In addition, FFFA and VFFA have a greater tendency to assemble into ordered nanostructures than KFFA, and VFFA prefers to form a large beta-sheet like structure from cluster analysis.

 Please wait while we load your content...
Please wait while we load your content...