Phase segregation in hydroxyfluorapatite solid solution at high temperatures studied by combined XRD/solid state NMR†
Abstract
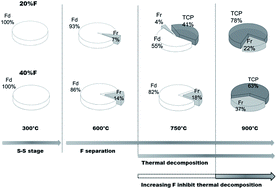
Fluoride substituted apatite is a key player among currently existing biomaterials. The 19F MAS-NMR is perhaps the most and only reliable technique to detect fluoride substitution in apatite. Typically any 19F MAS-NMR signal between −101.0 and −107.0 ppm is often used to identify fluoride substitution in apatite. Until now no explanation has been given as to why there is such a large variation in the NMR signals of these crystalline species. In this study, for the first time, we were able to explain this large variation in the 19F chemical shift values often seen in the literature. Hydroxyfluorapatites (FHA) with varied fluoride substitution and free from other substitutions have been synthesized via solution route followed by heat treatment in air at different temperatures up to 900 °C. Solid-state nuclear magnetic resonance (19F and 31P MAS-NMR) and X-ray diffraction (XRD) were used to characterize the synthesized powder samples. Formation of solid solution with varied hydroxyl/fluoride ratio was observed after the heat treatment up to a temperature of 300 °C. FHA samples decomposed to β-tricalcium phosphate (β-TCP) at higher temperature, which started from 20% F sample at 750 °C. With increasing F%, the FHA became more thermally stable and 80% F sample did not show β-TCP until 900 °C. An empirical nonlinear correlation between 19F NMR chemical shift and relative F% had been established. The mechanism of FHA solid solution formation and its thermal instability is proposed.

 Please wait while we load your content...
Please wait while we load your content...