Aggregation behavior of N-alkyl imidazolium-based poly(ionic liquid)s in an organic solvent†
Abstract
Poly(ionic liquid)s (PILs) have promising potential to form assemblies with ordered structures in organic media, but experimental investigation on the detailed aggregation behavior of PILs has seldom been involved. In this study, a series of N-alkyl imidazolium-based PILs with different chemical composition in their n-propanol solution was investigated by conductivity measurements. TEM images indicate that PILs with long alkyl chains exhibit onion-like multilamellar structures. The thermodynamic parameters suggest that aggregation of PILs in their good solvents (n-PrOH) is spontaneous, driven by entropy change associated with a hydrophobic effect. As increasing alkyl chain length from 8 to 16, the contribution of per alkyl chain to ΔGθm for PIL aggregation is found to decrease by more than 2 times. The nonsolvent (H2O) contribution to change of Gibbs free energy 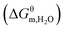 as well as critical aggregation concentration (CAC) decrease linearly with the volume fraction of water in mixed solvents, implying that PIL aggregation becomes more favorable with the addition of nonsolvent. The results mentioned above play an important role in understanding the aggregation process of PIL in an organic solvent and developing functional materials with well-ordered structures.
as well as critical aggregation concentration (CAC) decrease linearly with the volume fraction of water in mixed solvents, implying that PIL aggregation becomes more favorable with the addition of nonsolvent. The results mentioned above play an important role in understanding the aggregation process of PIL in an organic solvent and developing functional materials with well-ordered structures.

 Please wait while we load your content...
Please wait while we load your content...