Cysteine redox state plays a key role in the inter-domain movements of HMGB1: a molecular dynamics simulation study†
Abstract
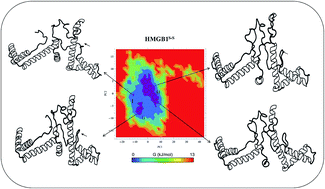
High mobility group box protein 1 (HMGB1) is an abundant, conserved, non-histone nuclear protein that can serve as an alarmin, driving the pathogenesis of inflammatory and autoimmune diseases. In addition to its intracellular functions, HMGB1 can be released to the extracellular environment where it mediates the activation of the innate immune response, resulting in chemotaxis and cytokine release. HMGB1 contains three conserved redox-sensitive cysteines (C23, C45, and C106), and modifications of these cysteines determine the bioactivity of extracellular HMGB1. To advance our understanding of the redox-dependent functional changes of HMGB1, we have modeled full-length HMGB1 and simulated three different states of the protein, including its C23A and C106A mutants. Principal component analysis suggests that redox states affect the disordered loop movements, and subsequently the domain movements, of the active B-box domain that determines the fate of cytokine activity. We have also explored the free energy landscape of the redox states of HMGB1 to understand their crucial structural differences. These findings may have identified redox-dependent features that enable functional conformational transitions. Furthermore, active HMGB1 was docked onto a complex of Toll-like receptor 4 and myeloid differentiation factor 2 to predict the interactions that may provide helpful insights into the potential role of HMGB1 as therapeutic target for numerous autoimmune diseases.

 Please wait while we load your content...
Please wait while we load your content...