A Pb2+-binding polychelatogen derived from thionated lactide†
Abstract
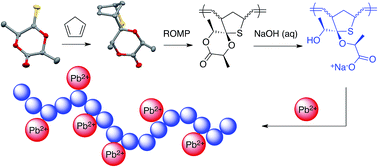
The synthesis and characterization of a polychelatogen derived from thionated lactide is reported. The four step synthesis from L-LA requires thionation and thia-Diels–Alder steps to afford a highly strained spiro-lactone adduct that is amenable to ring-opening metathesis polymerization. Saponification of the polymer affords a polyanion that exhibits a high affinity for toxic Pb2+ in aqueous solutions, results that we attribute to thioether and hydroxy carboxylate chelating moieties that are integrated into the polymer backbone and residues respectively.


 Please wait while we load your content...
Please wait while we load your content...