Catalytic properties of N-hydroxyphthalimide immobilized on a novel porous organic polymer in the oxidation of toluene by molecular oxygen
Abstract
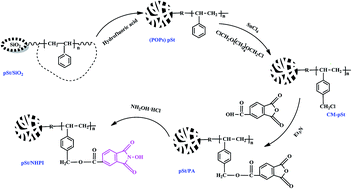
By molecular design, using a micro-sized macroporous silica mold, N-hydroxyphthalimide (NHPI) was synthesized and immobilized on a novel porous organic polymer (POP) polystyrene (pSt). The POP pSt was prepared using surface-initiated graft polymerization and the silica matrix was etched away. Subsequently, with 1,4-dichloromethoxybutane used as a chloromethylation reagent, a chloromethylated POP was obtained through a Friedel–Crafts alkylation reaction. Next, phthalic anhydride was bonded onto the POP through an esterification reaction, which was carried out between the chloromethyl groups on the POP and the carboxyl group of trimellitic anhydride (TMA), obtaining the product POP-PA. Then the bonded phthalic anhydride (PA) was reacted with hydroxylamine hydrochloride, and the NHPI-immobilized heterogeneous material pSt/NHPI was obtained. The products obtained were fully characterized. Finally, a co-catalyst system constituted of the solid catalyst pSt/NHPI and a small amount of Co(OAc)2 was used in an oxidation reaction process for toluene with molecular oxygen as the oxidant at a low temperature and a normal pressure of oxygen. The experimental results showed that the co-catalyst system can thoroughly oxidize toluene to benzoic acid, and this combination catalyst also possessed high catalytic activity, excellent catalytic selectivity and recyclability.

 Please wait while we load your content...
Please wait while we load your content...