Ethanol promoted titanocene Lewis acid catalyzed synthesis of quinazoline derivatives†
Abstract
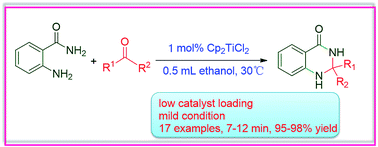
An efficient catalytic system involving in situ activation of kinetically inert titanocene dichloride with alcoholic solvent for the synthesis of quinazoline derivatives was developed. 1 mol% Cp2TiCl2 at 30 °C afforded 17 examples of quinazoline derivatives with yields of 95–98% in 7–12 minutes. Mechanistic experiments using in situ NMR and HRMS established that the coordination of ethanol to the titanocene moiety released the catalytic species [Cp2Ti(OCH2CH3)2].

 Please wait while we load your content...
Please wait while we load your content...