Effect of TiO2 morphology on the benzyl alcohol oxidation activity of Fe2O3–TiO2 nanomaterials†
Abstract
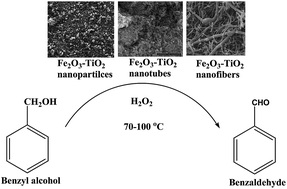
Three series of Fe loaded TiO2 anatase (1, 3, 5 and 7 mol% Fe) nanomaterials with different morphologies: nanoparticles (NP), nanotubes (NT) and nanofibers (NF) were synthesized and calcined at 400 °C. The physico-chemical properties of the catalysts were studied by using elemental analysis, XRD, UV-vis, N2-physisorption, SEM, TEM, XPS, pyridine adsorption using FTIR and H2-TPR techniques. It was observed that iron oxide was highly dispersed on the TiO2-NP support due to its strong interaction. The catalytic activity of the catalysts was tested in the oxidation of benzyl alcohol with hydrogen peroxide at mild reaction temperatures (70–100 °C) and atmospheric pressure. The highest activity was observed with 3 mol% Fe supported on TiO2-NP at 100 °C. It seems that TiO2-NP is unique in stabilizing small Fe2O3 nanoparticles. A greater number of surface hydroxyl groups in TiO2-NT and NF tend to increase the density of adsorption sites and/or the affinity of the surfaces with the Fe2O3 precursor. This appears to favor agglomeration, which results in a higher density of larger iron oxide particles. Fe-TiO2-NP catalysts display high activities due to a detrimental morphology effect, high surface area of the TiO2 support, dispersion of Fe2O3, more Lewis acid sites and easy reducibility of total catalysts. It was also observed that the Fe-TiO2 nanomaterials possessed two types of Fe3+ ions on the support surface; one dispersed in which Fe3+ ions interact with the TiO2 surface and another in which Fe2O3 crystals are located on the surface of the catalyst. The catalyst which possessed the former species exhibited the best performance in the oxidation of benzyl alcohol. Metal oxide leaching studies prove the true heterogeneous nature of the reaction. The catalysts are found to be reusable and resistant to rapid deactivation.

 Please wait while we load your content...
Please wait while we load your content...