Exfoliation of graphite and graphite oxide in water by chlorin e6†
Abstract
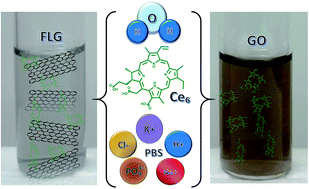
Few layer graphene (FLG) and graphene oxide (GO) are considered important materials for the development of future technological applications. Diverse strategies are followed for their synthesis and production resulting in graphene materials with different processability, electronic, optical, mechanical, and chemical properties. In particular, many efforts are directed at the integration of FLG or GO with water dispersable functional molecules. Recent advances in ultrasonication techniques have led to the control over the synthesis of carbon nanostructures using a versatile synthetic tool. Herein, we demonstrate the facile preparation of two different types of chlorin e6 (Ce6) nanohybrids in biocompatible media: few-layer graphene (FLG–Ce6) and graphene oxide (GO–Ce6) in deionized water (DW) and phosphate buffered saline (PBS). The exfoliation is energetically driven by acoustic cavitation while molecular interactions are responsible for the stabilization of FLG and GO in water by Ce6. The nanohybrid materials might find applications in energy and biomedicine fields since the main photophysical features of Ce6, such as its efficient use of energy in the near infrared region, its light harvesting properties, and its capacity for energy and electron transferring processes, are well preserved.

 Please wait while we load your content...
Please wait while we load your content...