Physicochemical properties of functionalized 1,3-dialkylimidazolium ionic liquids based on the bis(fluorosulfonyl)imide anion†
Abstract
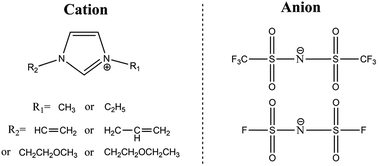
A new series of ether- or alkenyl-functionalized 1,3-dialkylimdazolium ILs based on the FSI anion were prepared and their physicochemical properties (melting point, thermal stability, viscosity, conductivity and electrochemical stability) were studied in detail and compared with the corresponding TFSI-based ILs. It was confirmed that introduction of ether or alkenyl groups and FSI anions jointly could reduce viscosity and enhance conductivity. These FSI-based ILs owned viscosities lower than 30 mPa s and conductivities higher than 7 mS cm−1. AEI-FSI had the lowest viscosity (17.4 mPa s) among all the reported FSI-based ILs and it had relatively higher conductivity (12.8 mS cm−1) as well. The electrochemical windows of most ILs were wider than 3.7 V, indicating their promising application for electrochemical devices.

 Please wait while we load your content...
Please wait while we load your content...