Novel fluorine-free 2,2′-bis(4,5-dimethylimidazole) additive for lithium-ion poly(methyl methacrylate) solid polymer electrolytes
Abstract
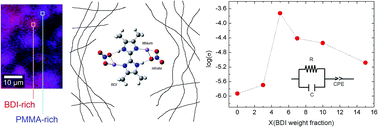
In this report, we study the effect of the addition of fluorine-free 2,2′-bis(4,5-dimethylimidazole) (BDI) on lithium-ion solid polymer electrolytes based on lithium nitrate and poly(methyl methacrylate) (PMMA). Confocal Raman spectroscopy and theoretical Raman calculations were performed to study lithium dissociation and the interaction mechanism of the lithium and nitrate ions with the BDI additive. Additionally, X-ray diffraction analysis showed that the mean coherence length of the PMMA chains is influenced by the BDI concentration. The incorporation of the BDI additive favors the dissociation of lithium salt leading to a maximum enhancement on the lithium conductivity of up to 1.88 × 10−4 S cm−1 for a critical BDI addition of ∼5% with respect to PMMA, which is very high in comparison with the conductivities of ∼10−4 S cm−1 usually observed for these solid polymer electrolytes.

 Please wait while we load your content...
Please wait while we load your content...