Self-healing of thermally-induced, biocompatible and biodegradable protein hydrogel†
Abstract
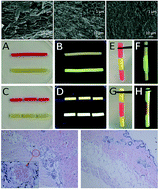
Serum albumin is the most abundant protein in the circulatory system to transport fatty acids, metabolites and drugs. In this study, a highly biocompatible protein hydrogel was prepared from bovine serum albumin (BSA) via thermal treatment. A circular dichroism study indicates that thermally-induced partial unfolding of the protein molecules exposes the buried hydrophobic groups in the core to the environment, thus leading to the formation of fine stranded 3-D networks. By controlling the heating temperature and protein concentration, the mechanical strength and structural stability of the as-prepared BSA hydrogel can be facilely manipulated. The moderate denaturation of the protein within the hydrogel system allows repetitive self-healing after damage when moderate heat was induced. The tensile strength and break strain of fully healed protein hydrogel were recovered to almost 100% of the original strength and elongation abilities. Additionally, the good biocompatibility of this hydrogel system was demonstrated through in vitro cytotoxicity analysis first. Furthermore, in vivo experiments using immunocompetent mice show that the subcutaneously injected hydrogel in mice can be fully degraded with negligible acute inflammatory response, indicating excellent in vivo biocompatibility. These features indicate that the as-developed self-healing protein hydrogel system with good biocompatibility and biodegradability holds great potential in the field of biomedical engineering.

 Please wait while we load your content...
Please wait while we load your content...