Tandem allylic alcohol isomerization/oxo-Michael addition reaction promoted by Re2O7†
Abstract
Re2O7 catalyzed tandem allylic alcohol isomerization/oxa-Michael addition reaction of cyclohexadienone was developed. The reaction features a regioselective and stereoselective isomerization of allylic alcohol and diastereoselective ring closure via oxa-Michael addition. This method also enabled construction of enantiopure bicyclic enones from substrates incorporated with chiral allylic alcohol via efficient chirality transfer.
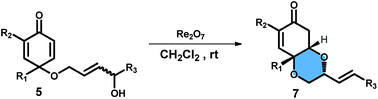

 Please wait while we load your content...
Please wait while we load your content...