Mechanism and free energy profile of base-catalyzed Knoevenagel condensation reaction†
Abstract
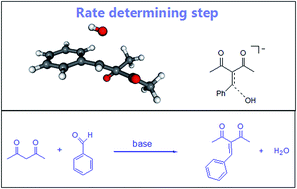
The Knoevenagel condensation reaction is a classical method for carbon–carbon bond formation. This reaction can be catalyzed by homogeneous or heterogeneous bases, and in the past ten years many different solid bases catalysts have been investigated. In this report, we have done a reliable theoretical analysis of the reaction mechanism and free energy profile of acetylacetone reaction with benzaldehyde catalyzed by methoxide ion in methanol solution. The analysis was extended for solventless conditions and solid base catalysis. We have found that the enolate addition to the benzaldehyde is a rapid step, contrary to general assumptions on the mechanism. The rate-determining step is the leaving of the hydroxide ion from the anionic intermediate, with a predicted overall free energy barrier of 28.8 kcal mol−1. This finding explains the experimental observation that more polar medium favor the reaction, once the transition state is product-like and involves the formation of the highly solvated hydroxide ion. The present results provides useful insights on this reaction system.

 Please wait while we load your content...
Please wait while we load your content...