Brookite TiO2 photocatalyzed degradation of phenol in presence of phosphate, fluoride, sulfate and borate anions†
Abstract
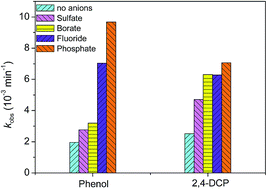
The effects of common inorganic anions on the photocatalytic degradation of organic pollutants over anatase and rutile TiO2 in aqueous solution have been widely investigated in the literature. In this work, we report a positive effect of phosphate, fluoride, sulfate and borate anions on the photocatalytic degradation of phenol and 2,4-dichlorophenol over brookite TiO2 in aqueous suspensions. In all cases, the rate of phenol degradation over brookite was first-order. Such anion-induced rate enhancement of phenol degradation changed with the initial pH and anion concentration of the suspension, and had a positive correlation with the amount of anions adsorbed on brookite. Moreover, these anions also showed a positive effect on the formation of coumarin–OH adduct, a fluorescent probe of OH radicals. However, upon the addition of these anions, the brookite photocatalyzed formation and decomposition of H2O2 in aqueous suspensions were all inhibited, ascribed to decrease in the amount of O2 and H2O2 adsorption on brookite, respectively. A possible mechanism responsible for the observed effect of these anions is discussed.

 Please wait while we load your content...
Please wait while we load your content...