Role of salt in the aqueous two-phase copolymerization of acrylamide and cationic monomers: from screening to anion-bridging†
Abstract
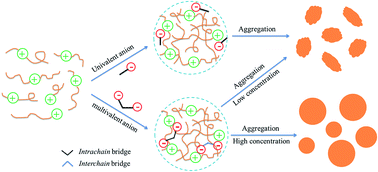
The aqueous two-phase copolymerization (ATPP) of acrylamide and cationic monomers was carried out in poly(ethylene glycol) (PEG) aqueous solution. The effects of inorganic salts on the ATPP process were systematically investigated and obvious differences were observed between univalent and multivalent anions. For the ATPP system with added univalent anions such as Cl−, the critical conversion for the phase separation decreased as the particle size increased, but the polymerization kinetics and final molecular weight of the copolymers were similar with that of the salt-free system. However, after adding multivalent anions such as SO42− into the ATPP system, the critical conversion for the phase separation decreased sharply, the polymerization rate slowed down, the final molecular weight of the copolymers increased, and the particle size greatly increased. Moreover, the system underwent a unique particle formation process. A mechanism for the role of anions in the ATPP process was proposed. The salting-out effects of the univalent anions screen the electrostatic repulsion between cationic species and lead to the formation of gravel-like particles. But for multivalent anions, when the concentration is low, their salting-out effect is involved in the particle formation process and results in the formation of gravel-like particles as well. When the concentration becomes high, their bridge effect is dominant, making large and spherical particles appear.

 Please wait while we load your content...
Please wait while we load your content...