Metalized B40 fullerene as a novel material for storage and optical detection of hydrogen: a first-principles study
Abstract
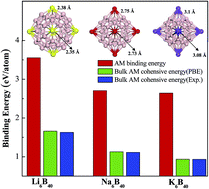
The first experimentally observed all-boron fullerene (B40) has potential applications in hydrogen storage [Nat. Chem., 6, 727 (2014)]. The surface of B40 fullerene contains 4 heptagonal rings and 2 hexagonal rings, which may facilitate metal atom adsorption. Based on (van der Waals corrected) density functional theory, we show that alkali metal (AM) adsorbed on B40 can serve as a promising candidate not only for hydrogen storage, but also for hydrogen detection. The binding energy of AM atoms on B40 strengthens, due to the energetically favorable B 2p-AM p orbital hybridization. Thus, AM atoms are not likely to form clusters on B40, indicating a good reversible hydrogen storage. The (AM)6B40 can reach a gravimetric capacity of ∼8 wt% hydrogen with an optimal adsorption energy that is intermediate between the physisorbed and chemisorbed state. Interestingly, the number of adsorbed H2 molecules per AM atom depends on the position of the AM atoms adsorbed on the B40 surface. Furthermore, the optical absorption spectra of (AM)6B40 before and after hydrogen molecule adsorption are computed and a clear red (blue) shift is observed. These results can serve as a guide in the design of promising hydrogen storage materials and optical sensors based on the B40 fullerene.

 Please wait while we load your content...
Please wait while we load your content...