A novel C–C radical–radical coupling reaction promoted by visible light: facile synthesis of 6-substituted N-methyl 5,6-dihydrobenzophenanthridine alkaloids†
Abstract
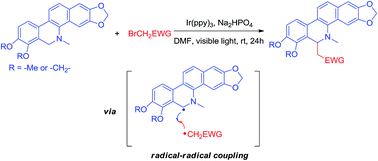
A novel photoredox-mediated direct intermolecular C–H functionalization of N-methyl 5,6-dihydrobenzophenanthridine is developed utilizing the visible light-induced reductive quenching pathway of photocatalyst Ir(ppy)3. In the proposed coupling mechanism, an α-amino C-radical is generated at the 6-position of N-methyl 5,6-dihydrobenzophenanthridine which is capable of coupling with α-EWG (electron withdrawing group) substituted C-radicals. The utility of this methodology has been demonstrated via rapid access to the analogue of natural 6-substituted N-methyl 5,6-dihydrobenzophenanthridine alkaloids.

 Please wait while we load your content...
Please wait while we load your content...