The influence of ionic liquid additives on zinc half-cell electrochemical performance in zinc/bromine flow batteries
Abstract
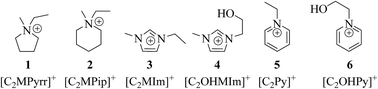
Six ionic liquids were assessed for their suitability as alternative bromine-sequestering agents (BSAs) in zinc/bromine redox flow batteries (Zn/Br RFBs) via comparison against conventional BSA, 1-ethyl-1-methylpyrrolidinium bromide ([C2MPyrr]Br). These alternative BSAs included the bromide salts of the following cations: 1-ethyl-1-methylpiperidinium ([C2MPip]+), 1-ethyl-1-methylimidazolium ([C2MIm]+), 1-(2-hydroxyethyl)-3-methylimidazolium ([C2OHMIm]+), 1-ethylpyridinium ([C2Py]+) and 1-(2-hydroxyethyl)pyridinium ([C2OHPy]+). Cyclic and linear sweep voltammetry, as well as electrochemical impedance spectroscopy, were performed to understand the influence of electrolytes containing these ionic liquids on zinc half-cell electrochemical performance. Solutions with [C2Py]Br, [C2MIm]Br and [C2OHPy]Br improved zinc half-cell performance (highest-magnitude current, charge, maximum power and energy) when compared to those utilizing [C2MPyrr]Br. Electrolytes employing these BSAs also reduced the nucleation overpotential of zinc electrodeposition and stripping compared to those using [C2MPyrr]Br. Zinc electrodeposits obtained during charging from electrolytes containing the different BSAs were analyzed via scanning electron microscopy and X-ray diffraction. Scanning electron micrographs showed a strong relationship between the chemical structure of the BSA employed and the crystallinity of zinc electrodeposits, with solutions containing [C2OHMIm]Br, [C2Py]Br and [C2OHPy]Br producing more compact zinc deposits than those with other BSAs. These findings warrant further investigation of BSAs with delocalized cationic charge. While these compounds have been proposed for application in Zn/Br systems, they are also potentially adaptable to other types of RFBs, which employ the Br2/Br− redox couple and use electrolytes containing BSAs.


 Please wait while we load your content...
Please wait while we load your content...