Accelerated synthesis of MnO2 nanocomposites by acid-free hydrothermal route for catalytic soot combustion†
Abstract
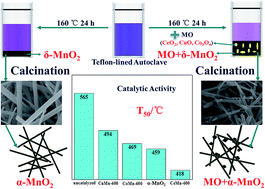
MnO2 nanocomposites with porous structure were successfully synthesized by a facile hydrothermal route from KMnO4 without the addition of any acid. Among CeO2, CuO and Co3O4, an accelerated synthesis of MnO2 assisted by CeO2 was observed, which contributed to the redox transformation between Ce3+ and Mn7+. The as-prepared catalysts were characterized by XRD, N2 adsorption–desorption, ICP, SEM, TEM, H2-TPR, and XPS. Lattice shrink occurred after the hydrothermal reaction of CeO2 with KMnO4, while lattice expansion in CeO2 was observed after calcination at high temperature. The above phenomena are ascribed to the conversion between small sized Ce4+ and large sized Ce3+ in CeO2. The concentration of surface Ce3+ decreases after the hydrothermal reaction with KMnO4. The greater proportion of Ce3+ after calcination results in more oxygen vacancies that are beneficial for the catalytic oxidation of soot. The best catalytic performance is acquired over the CeMn-600 catalyst and the corresponding T10, T50, and Tm,l are 320 °C, 418 °C and 420 °C, respectively. The strong interaction between the CeO2 and MnO2 is also a key factor for outstanding catalytic activity for soot combustion.

 Please wait while we load your content...
Please wait while we load your content...