Promising hydrogen storage properties of cost-competitive La(Y)–Mg–Ca–Ni AB3-type alloys for stationary applications
Abstract
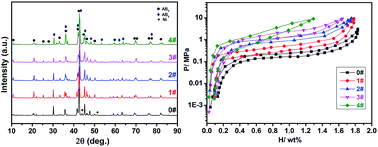
In this paper, La(0.65−x)YxMg1.32Ca1.03Ni9 (x = 0, 0.05, 0.20, 0.40, 0.60) alloys were prepared by an induction melting method, and the effects of Y substitution on the hydrogen storage properties were systematically investigated. The results showed that the hydrogen sorption plateau pressures could be easily tailored by altering the Y amount, and the effective hydrogen desorption capacities at 0.1 MPa H2 below 80 °C could be greatly promoted with the optimal amount of Y substitution. In addition, the cost of the prepared alloys is quite low due to the absence of high price rare earth metals. Therefore, the cost-competitive La(Y)–Mg–Ca–Ni AB3-type alloys prepared in this study successfully meet the demands of proton-exchange membrane fuel cells and other stationary apparatus, showing broad application potential.

 Please wait while we load your content...
Please wait while we load your content...