Diversity oriented synthesis of β-carbolinone and indolo-pyrazinone analogues based on an Ugi four component reaction and subsequent cyclisation of the resulting indole intermediate†
Abstract
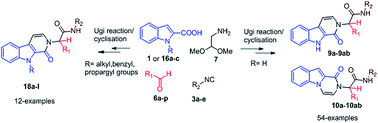
A one-pot domino multicomponent reaction for the rapid, integrated and versatile synthesis of highly functionalized β-carbolinones (9a–9ab) and indolo-pyrazinones (10a–10ab) has been established. The reaction involves an Ugi-four component reaction of indole 2-carboxylic acid (1 or 16a–c), aryl or alkyl aldehyde (6a–p), isocyanide (3a–e), and aminoacetaldehyde dimethyl acetal (7) followed by in situ acid-mediated deprotection/activation/electrophilic cyclisation/and aromatisation. The products were obtained from Ugi adducts in good to excellent yield within short periods of 20–30 min at room temperature (35 °C). The potential of the methodology is proved by development of a diverse library of heterocyclic compounds with point and skeletal diversity. Moreover, the synthesis has also been accomplished using N-substituted derivatives of indole 2-carboxylic acid that delivers highly diverse N-substituted β-carbolinone (18a–l) heterocycles of medicinal importance.
- This article is part of the themed collection: Elegant Synthetic Routes to Indole Derivatives

 Please wait while we load your content...
Please wait while we load your content...