Artificial extracellular matrix delivers TGFb1 regulating myofibroblast differentiation
Abstract
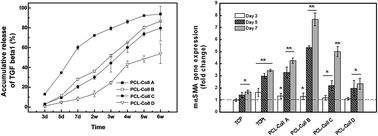
During wound healing, the contractile activity of myofibroblasts differentiated from fibroblasts in active transforming growth factor β1 (TGFβ1) conditions has vital functions. In wound dressing biomaterials, it is crucial to mimic the extracellular matrix to deliver the right amount of TGFβ1 in a spatiotemporally controlled manner. We report here, for the first time, a zero-order, sustained TGFβ1 release from electrospun biomimetic nanofibers realizing optimal cell viability and myofibroblast differentiation capacity, confirmed by cell metabolic activity CCK assay, gene expression level through real-time PCR and protein expression level through immunochemical staining.

 Please wait while we load your content...
Please wait while we load your content...