DFT insights into the cycloisomerization of ω-alkynylfuran catalyzed by planar gold clusters: mechanism and selectivity, as compared to Au(i)-catalysis†
Abstract
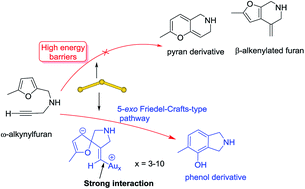
A detailed reaction mechanism of the triatomic gold cluster-catalyzed cycloisomerization of ω-alkynylfuran was systemically investigated via density functional theory at the TPSSh/def2-TZVP level. The computational results indicated that the 5-exo Friedel–Crafts-type mechanism is the most favorable mechanism to form the phenol derivatives. The strong interaction between the gold and vinyl fragments in the Friedel–Crafts adduct is essential for the priority of the 5-exo Friedel–Crafts-type mechanism. Then, the 5-exo Friedel–Crafts-type mechanism on the various planar gold clusters (Au4–10) was studied to clarify the size-effects of the planar gold clusters catalyzed ω-alkynylfuran cycloisomerization. The appropriate interactions between the alkyne group in the substrate and gold clusters play a key role for the 5-exo cyclization step. The energy barriers of the ring-closure of the dienone carbene–gold intermediate step show an interesting “odd–even” behavior respective to the number of gold atoms. The Au3 and Au4 clusters are the most active catalysts for the ω-alkynylfuran cycloisomerization to the phenol derivative. We also found that the active catalyst of the ω-alkynylfuran cycloisomerization catalyzed by the gold(I) complexes should be the gold(0) complexes of the in situ generation. The catalytic activity of the gold(0) complex is comparable with that of the planar gold clusters. These findings may guide the rational design of highly active gold catalysts for the ω-alkynylfuran cycloisomerization to phenol derivatives.

 Please wait while we load your content...
Please wait while we load your content...