Investigation of Ce(iii) promoter effects on the tri-metallic Pt, Pd, Ni/MgO catalyst in dry-reforming of methane
Abstract
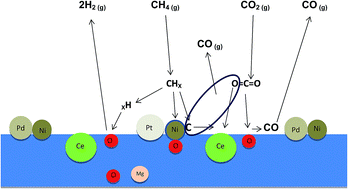
A mixture of cerium oxide and magnesium oxide supports with certain mole ratios of Mg2+/Ce3+ were prepared via the co-precipitation of Mg and Ce nitrates, and followed by impregnation with 1 wt% each of Ni, Pd, and Pt metals to form Pt, Pd, Ni/Mg1−xCexO catalysts. Evaluation of the prepared catalysts was carried out by a DRM reaction for 200 h and they were characterised by means of in situ XRD, XRF, XPS, BET, H2-TPR, TEM and TGA. It was found that the interaction of a suitable amount of MgO with Ce2O3 stabilised a cubic phase in the catalysts, which has a high basicity to adsorb CO2 forming a monoclinic Ce2O2CO3 species in the DRM reaction. The introduction of MgO also created surface oxygen ions. The oxidisation and the removal of the deposited carbon maybe achieved by both monoclinic Ce2O2CO3 and surface oxygen, keeping the metal Ni, Pd, and Pt catalyst at high activity and stability. The Ce2O3 as a promoter in the catalyst had several effects such as: stabilisation of the magnesia cubic phase; increase in its thermal stability, increase in the basicity of the support, decrease in the carbon deposition, and decrease in the reducibility of the Ni2+, Pd2+, and Pt2+ ions.

 Please wait while we load your content...
Please wait while we load your content...