Synthesis and phosphatase inhibitory activity of 3-alkynylestrones and their derivatives†
Abstract
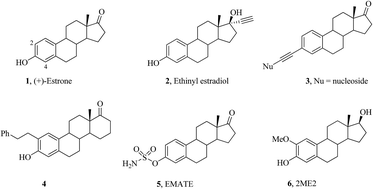
A range of 3-alkynylated 3-deoxy-estrones were prepared by Sonogashira reactions and transformed into estrone derived diones and quinoxalines. The alkynylated estrones and their derivatives exhibit significant biological activity as alkaline phosphatase inhibitors. The mode of action was illustrated based on docking studies.

 Please wait while we load your content...
Please wait while we load your content...