Explaining stability of transition metal carbides – and why TcC does not exist†
Abstract
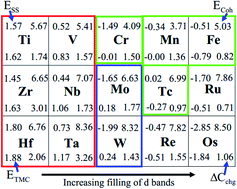
We analyze the formation of transition metal (TM) carbides, as determined by the strength of TM–TM and TM–C bonds, as well as lattice distortions induced by C interstitials. With increasing filling of the d-band of TMs, TM–C bonds become increasingly weak from the left of the periodic table to the right, with fewer and fewer C atoms entering the TMs lattice. Technetium (Tc) turns out to be a critical point for the formation of carbides, guiding us to resolve a long-standing dispute. The predicted Tc carbides, agreeing with measured X-ray absorption spectra, should decompose to cubic Tc and graphite above 2000 K. Consequently, we show that what has been claimed as TcC (with rocksalt structure) is actually a high-temperature cubic phase of elemental technetium.

 Please wait while we load your content...
Please wait while we load your content...