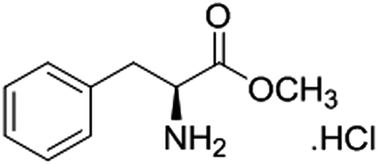
l-Phenylalanine methyl ester hydrochloride as a green corrosion inhibitor for mild steel in hydrochloric acid solution and the effect of surfactant additive
Abstract
The inhibitive effect of L-phenylalanine methyl ester hydrochloride (PMEH) on the corrosion of mild steel (MS) in 1 M HCl solution was investigated at different concentrations and temperatures (30–60 °C) by using weight loss measurements, potentiodynamic polarization measurement, electrochemical impedance spectroscopy (EIS), UV-visible spectrophotometry, and quantum chemical calculations. The obtained results revealed that PMEH is a good inhibitor for the corrosion of MS in HCl solution. The inhibition efficiency (IE) increased with the inhibitor concentration up to 400 ppm as well as with a rise in temperature, which is suggestive of a chemical adsorption mechanism. The adsorption of inhibitor onto the MS surface was found to obey the Langmuir adsorption isotherm. Thermodynamics of adsorption such as enthalpy of adsorption (ΔH), entropy of adsorption (ΔS), equilibrium of adsorption (Kads), Gibbs free energy (ΔG°), activation and synergism parameters (S1) were calculated and discussed. Corrosion attack morphologies as observed by scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX) qualitatively verify the results obtained by gravimetric as well as electro-chemical methods. The results obtained from all the techniques are in good agreement.

 Please wait while we load your content...
Please wait while we load your content...