Redox behavior, chromatographic and spectroscopic characterization of some reactive π-conjugated 4′-tricyanovinylhydrazone dyes
Abstract
A series of π-conjugated nonlinear 4′-tricyanovinylhydrazone (TCH) dyes was synthesized and characterized. The redox behaviors of the TCH dyes were studied by direct current (DC), and derivative polarography (DP), cyclic voltammetry (CV) and controlled potential coulometry (CPC). Excluding the nitro derivatives, the DC and DP polarograms of the compounds at pH < 5 showed two irreversible waves (peaks) corresponding to reduction of the ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– and the hydrazone (
C– and the hydrazone (![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
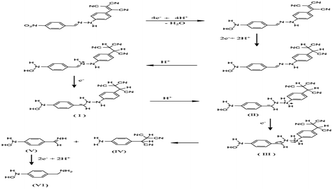
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) groups. The DC and DP polarography of the nitro derivatives at pH < 5 showed three waves (peaks). Plots of cathodic peak current (ip,c) vs. square root of scan rate (ν1/2) and log ip,c vs. log ν at −0.59 and −0.93 V at pH 4.46 were linear. The kinetic parameters (log kof,h, ΔG* and D) of the compounds were determined. Poor correlations for substituents on the E1/2 of the dyes with the Hammett substituent constants were noticed. Based on the chromatographic separation, spectroscopic characterization and molecular weight determination of the end products of the electrolyzed species of 4′-nitrotricyanovinylhydrazone, an electrochemical reduction mechanism is proposed.
N–) groups. The DC and DP polarography of the nitro derivatives at pH < 5 showed three waves (peaks). Plots of cathodic peak current (ip,c) vs. square root of scan rate (ν1/2) and log ip,c vs. log ν at −0.59 and −0.93 V at pH 4.46 were linear. The kinetic parameters (log kof,h, ΔG* and D) of the compounds were determined. Poor correlations for substituents on the E1/2 of the dyes with the Hammett substituent constants were noticed. Based on the chromatographic separation, spectroscopic characterization and molecular weight determination of the end products of the electrolyzed species of 4′-nitrotricyanovinylhydrazone, an electrochemical reduction mechanism is proposed.

 Please wait while we load your content...
Please wait while we load your content...